Do You Suffer from Notalgia Paresthetica?
Notalgia Paresthetica Clinical Trial in Alabama
Notalgia Paresthetica is a very common condition that is often underdiagnosed. If you suffer from extreme back itching, burning, or prickly-sensations, you may have this condition. Luckily, there are ways to treat Notalgia Paresthetica and one of them is by participating in a clinical trial to take part in the newest, most cutting-edge treatment for this condition. Keep reading to learn more about the Notalgia Paresthetica Clinical Trial and how to sign up today and get qualified as a participant.
Do You Have Notalgia Paresthetica?
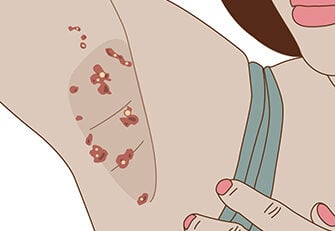
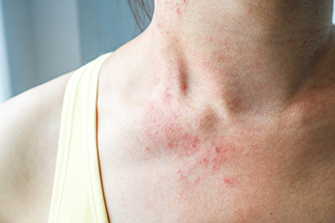
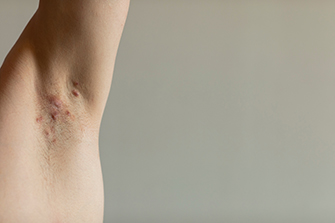
Notalgia Paresthetica is a common condition that predominately affects adults. The main symptom is intense itching, burning, or a tingly feeling present along the inner part of the shoulder blade and the spine. Because of the constant rubbing in this area most patients develop a colored patch. The most common cause of this condition in adults is pressure on a nerve root that gives sensation to the area. If this condition occurs in younger children it can be a sign of a hereditary disorder called Multiple Endocrine Neoplasia 2A.
Treatment Options for Notalgia Paresthetica
One of the most challenging parts about suffering from Notalgia Paresthetica is that it can be difficult to treat. Some of the most common treatment options to decrease symptoms of this condition include:
- Topical capsaicin cream or patch, however, most patients had symptoms return once stopping the medication.
- Topical steroids
- Pramoxine
- Lidocaine/prilocaine mixture
- Acupuncture
- Narrow band UVB
- Transcutaneous electrical nerve stimulation
- Spinal nerve block
- Osteopathic manipulation
- Notalgia Paresthetica Clinical Trial
What is the Notalgia Paresthetica Clinical Trial?
Do you want to try something new to help ease your NP symptoms? You may be the perfect fit for our clinical trial! Cahaba Clinical Research is currently conducting a clinical trial for a brand new, investigational treatment for Notalgia Paresthetica, called Difelikefalin. If you are 18 years of age or older and have moderate to severe itch due to this condition, we want to hear from you!
Here’s what participants can expect:
- Taking an oral study treatment twice daily. You will have a 3 out of 4 (75%) chance of receiving the investigational treatment rather than placebo (pill that does not contain any treatment) for the 8 weeks of treatment. You will be provided with all study medication, examinations and medical care related to the study at no cost to you.
- In-person visits. After the 8 weeks, there is a 2-week discontinuation period where you will have stopped taking the treatment. You will have a total of 7 in-person visits, which take approximately 30 minutes to 2.5 hours each, except for the screening visit which may take up to 3 hours.
- Approximately 15 weeks. Participation will last approximately 15 weeks, including up to 4-weeks for screening and a 2-week post-treatment follow-up visit.Compensation for time and travel may be available.
For more information on Cahaba Clinical Research, or to see a list of other clinical trials we are currently enrolling participants in, visit us at CahabaClinicalResearch.com!